Introduction
Pharmacovigilance is one of the fastest-growing areas in pharmacy. It focuses on detecting, assessing, understanding, and preventing adverse effects or any other drug-related problems. For B. Pharmacy students, pharmacovigilance is important not only as a subject concept, but also as a strong career pathway in clinical research and the pharmaceutical industry.
What is Pharmacovigilance?
Pharmacovigilance means drug safety monitoring. It ensures that medicines available in the market remain safe for patients by continuously tracking:
- Adverse Drug Reactions (ADRs)
- Side effects and toxicity
- Drug interactions
- Medication errors
- Quality complaints related to drugs
- Special situations such as pregnancy, lactation, pediatrics, and geriatrics
In short, pharmacovigilance works to maintain the balance between benefit and risk of a medicine throughout its life cycle.
Why Pharmacovigilance is Important in Pharmacy
- Some side effects appear only after a drug is used by a large population
- Real-world patients often take multiple medicines together
- Early detection of ADRs prevents serious harm and saves lives
- Supports safe prescribing and proper patient counseling
- Helps regulatory authorities take actions like warnings, label changes, or drug withdrawal
Key Terms Every B. Pharma Student Should Know
- ADR (Adverse Drug Reaction): Harmful and unintended response to a drug at normal doses
- AE (Adverse Event): Any unwanted medical occurrence after drug use
- SAE (Serious Adverse Event): Event causing death, hospitalization, disability, or life-threatening condition
- ICSR (Individual Case Safety Report): Detailed report of a single patient ADR case
- Signal Detection: Identification of new or unknown safety issues of a drug
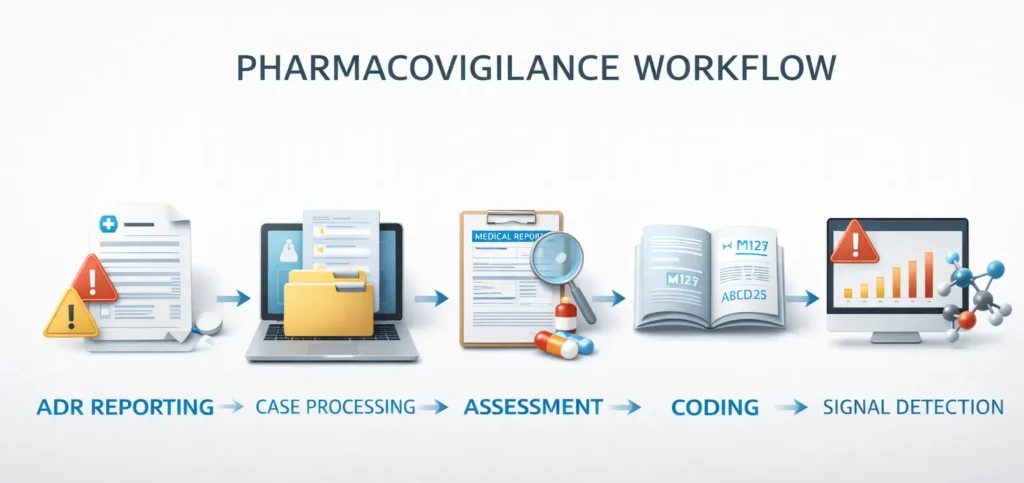
Pharmacovigilance Process Step-by-Step
- ADR Collection and Reporting
- Adverse drug reactions are reported by healthcare professionals, patients, hospitals, and pharmaceutical companies using reporting forms, hospital records, mobile apps, and online portals.
- Case Processing
- Each report is checked for patient details, suspected drug, reaction description, dose, route, duration, seriousness, and outcome.
- Case Assessment
- The reported case is evaluated to determine causality, seriousness, and whether the reaction is expected or unexpected.
- Data Entry and Medical Coding
- Standardized medical dictionaries such as MedDRA and WHO Drug Dictionary are used to code adverse events and medicines.
- Signal Detection and Risk Management
- Safety data is analyzed to identify patterns or new risks. Based on findings, actions such as label updates or warnings may be implemented.
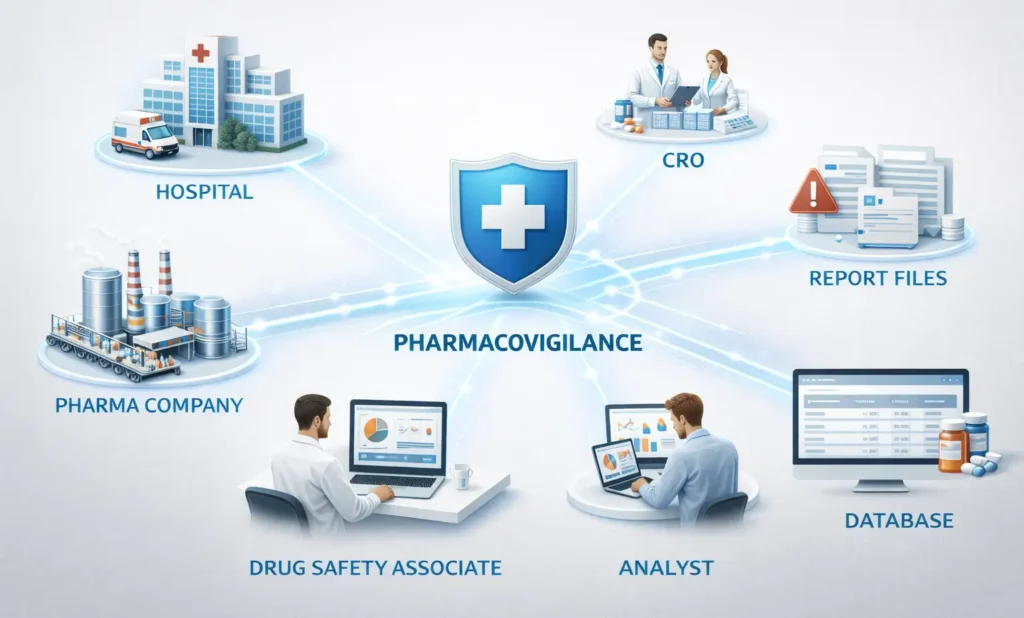
Career Scope in Pharmacovigilance After B. Pharmacy
- Drug Safety Associate
- Pharmacovigilance Executive
- Case Processing Associate
- Medical Reviewer
- Signal Detection Analyst
- Risk Management Specialist
- Pharmacovigilance Quality Specialist
Pharmacovigilance professionals can work in pharmaceutical companies, CROs, clinical research organizations, and drug safety BPO units.
Skills Needed to Start a Career in Pharmacovigilance
- Strong basics of pharmacology and ADR concepts
- Good English writing and documentation skills
- High attention to detail and accuracy
- Basic understanding of clinical research
- Knowledge of ICSR preparation and reporting
- Basic computer and Excel skills
Recommended Courses and Certifications
- Pharmacovigilance certification course
- Clinical research and GCP training
- Regulatory affairs fundamentals
- Introductory MedDRA training
Conclusion
Pharmacovigilance is a high-scope and stable career option for B. Pharmacy students interested in drug safety and clinical research. With proper knowledge, skill development, and training, graduates can enter this field and grow into advanced roles within the pharmaceutical industry.

